Unformed Patent Challenge Procedures
On May 12, 2017, the CFDA (now the CDA) issue an “Announcement of the China Food and Drug Administration on Soliciting Opinions on the Relevant Policies on Encouraging Innovation in Drug and Medical Equipment and Protecting the Rights and Interests of Innovators” (the “Announcement”) for public comments. The Announcement appears to provide specific linkage mechanisms similar to the U.S. Hatch- Waxman Act, though it has yet to be promulgated. The detail of the patent challenge procedures provided in the Announcement is as follows:
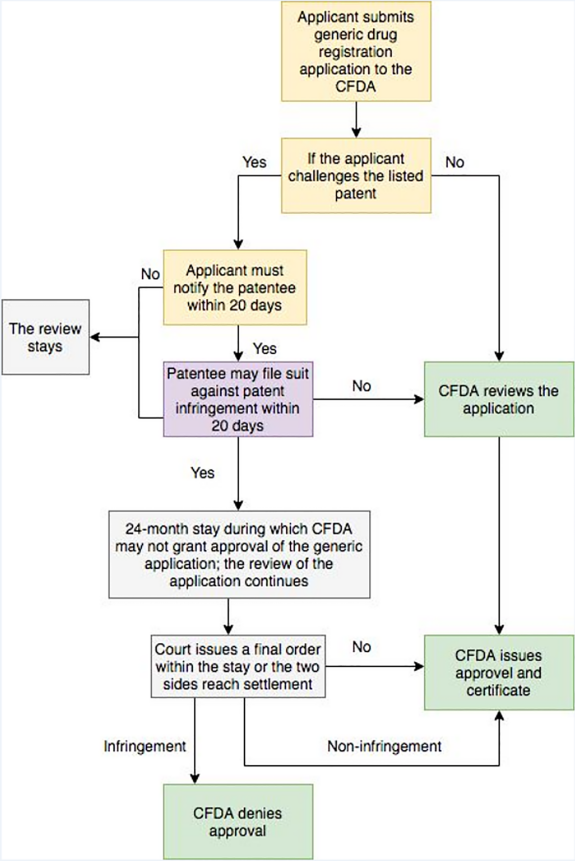
The Hatch-Waxman Act divides a generic drug applicant’s statement into four categories14, whereas Chinese patent law has no more detailed regulations. In the U.S., a Paragraph IV certification is a potential trigger for Hatch-Waxman Act patent litigation because the filing of an application with that certification is a statutory act of patent infringement (35 U.S.C.§ 271(e)(2))15. That is, for a drug claimed in a patent or the use of which is claimed in a patent, if the purpose of such submission is to obtain approval, to engage in the commercial manufacture, use, or sale of the drug before the expiration of such patent, that is one statutory act of patent infringement (so called “artificial infringement”). As abovementioned, an unsolved problem for China is the lack of a legal basis to claim patent infringement against a generic company that applies for regulatory approval for a drug that is still covered by a third-party patent, due to the act of filing for drug regulatory does not constitute an act that infringes a patent. Consequently, pre-trial injunction relief stipulated in Article 66 of the Patent Law is also not available, as a precondition of pre-trial injunction is that the action constitutes infringement. Though there has been calls to amend the Patent Law to cover the act of applying for drug regulatory approval, even the most recent draft amendment of the Patent Law, which was submitted to the National People's Congress for review, provides no relevant amendment. It remains to be seen how the authorities will address this gap in any potential new drug patent linkage system for China.
In addition, the “24-month stay” period provided in the “Announcement” may have little or no value in practical terms, when considering the time required to determine patent infringement proceedings in China in relation to pharmaceutical. The statistical data of Beijing IP court suggest that the current average trial period is 539 days for the first instance of invention patent infringement litigation concluded in 201716. Unlike the US where the questions of infringement and validity are heard together in a patent infringement lawsuit, the Chinese patent action may likely be a bifurcated proceeding in which infringement and validity are dealt with by separate tribunals. Thus, during the patent infringement litigation process, a suspension may be granted depending on the discretion of the court due to the involved invalidation procedure. Once the patent infringement cases are suspended by the Court, the average trial period for the first instance of patent civil cases concluded from 2013 to 2017 has increased to 447 days17. However, these statistics refer to patent infringement proceedings in relation to all types of patents, and pharmaceutical patent litigation tends to take longer than two years.
Furthermore, jurisdictional oppositions, technical appraisal, as well as the technical complexity of the drug patent will also contribute to the overwhelming likelihood that the courts will fail to issue a decision in relation to patent infringement within the 24-month-stay period. Before the conclusion on infringement issues is reached, even though it is allowed to proceed with the drug application after the 24-month-stay expires, generic drug companies would remain cautious about potential infringement liabilities. Because with the amount of compensatory damages for patent infringement treading higher annually, a cost benefit analysis may not lead a generic manufacturer to conclude that that cost of infringement is negligible compared to the potential profit of a generic drug that is approved for market sale.
Another question here is, if a conclusion of infringement is reached at the time of filing drug application, it remains unclear whether any compensation is available and how to calculate any potential damages award. If there is no compensation, the patentee may not necessarily have the incentive to stop the generic drug at the drug application stage. Under the premise that patents of the branded drug are still in force, generic companies can still legally market generic drugs if either of the two following conditions are fulfilled:
the referenced patent is determined to be invalid;
or B.the generic drug does not infringe that patent.
Therefore, for a comprehensive drug patent linkage system, the applicant for a generic drug should also have the right to challenge the validity of a patent right, as well as secure a declaration of noninfringement.
The Announcement provides that “if relevant drug patent holders deem that such applicants have infringed their patents, they shall bring a patent infringement suit to the judicial authorities within 20 days upon receipt of their notice and notify the drug appraisal institutions at the same time”. If the patent holder doesn’t exercise the right of action timely, we believe that a generic pharmaceutical company is entitled to file litigation for noninfringement upon completion of the notice procedure18. However, the precondition for a non-infringement declaration is also that the particular action constitutes infringement.
If the patent invalidation is filed during the drug application stage, similar consideration arises that the dispute resolution period seems to be longer than 24 months. The average trial period for invalidation procedures before the Patent Reexamination Board is 200 days19.
According to available statistics, the average trial period is 294 days for the first instance of patent administrate litigation that concluded from 2013 to 2017, and the average trial period for the second instance patent administrate litigation concluded from 2013 to 2017 is 185 days20. Taking Beijing IP court for another example, the current average trial period is 594 days for the first instance of invention patent invalidation litigation concluded in 2017, while this number is 356 days in 2016; And the overall rate of revocation in the first instance was 12.5% in 2016 and 13.1% in 201721.
Unfortunately, if patent infringement litigation is intertwined with invalidation procedures, infringement disputes will roll back and forth between the court and the Reexamination Board. As a consequence, disputes will become tedious and the so-called patent link system will fail to serve its intended purpose. Therefore, it would appear that shortening the dispute resolution time period will be essential for a robust patent linkage system. Some potential steps that may be taken to shorten the dispute resolution period include the following:
Exclusive jurisdiction could be regulated to avoid delay arising out of objections to jurisdiction;
During the patent challenge procedure, the applicant for a generic drug should have the priority right to invalidate the patent in the drug application, and in order to facilitate the quick resolution of the dispute, if the generic applicant failed to file invalidation within a reasonable period, invalidation is not allowed in the patent infringement lawsuit filed by the patentee afterwards during drug application;
The court could establish a talent pool of technical experts together with the Reexamination Board and CDA;
The conclusion of the first instance invalidation case could be a reference for whether or not to resume the proceeding to the next step, considering the relative low revocation rate of the first trial. If the patent is invalidated in the first instance though it is not final, the application could be approved and a proper bond could be required by the CFA which would help compensate new drug companies and make damages awards more predictable for generic drug companies;
E.The conclusion of the first instance infringement case could be a reference for whether or not to approve the application. Under this circumstance, it is important for generic drug companies to keep infringement damages within a reasonable range, for example, patent infringement liability insurance could be considered to import into the drug patent system, etc.
Experimental Data Exclusivity Period
The CDA recently issued a draft Implementing Measures for Protection of Trial Data of Drugs (for Provisional Implementation) (“Draft Implementing Measures”) on April 25, 2018 for public comment.
The Draft Implementing Measures specifies the scope of data protection scope, extends some data protection periods, and provides a proposed procedure for applying for and granting a data protection right. Basically, it provides that innovative drugs approved to enter the domestic market will be entitled to a data protection period of six years, and this data protection period will be doubled to 12 years for innovative biological products for curative uses22. Before the expiration of the data protection period, the CFDA will not approve any applications for the same category of drugs filed by other applicants without the consent of the data protection right holders, unless the clinical-trial data is obtained by the other applicant on their own or they have obtained approval from the license holder.
The data protection system can prevent the approval of generic drugs, serving as a powerful supplement to the patent protection system. Especially for biological drugs, the trial cost of which is much higher.
However, for generic drugs to which pertinent patents have been invalidated (i.e., first-to-market generics), the data exclusivity period is not provided in the “Draft Implementing Measures”23.
The greatest attraction of the patent challenge system is that the monopoly of the market for the first generic drugs, which will greatly encourage generic pharmaceutical companies to challenge patents. The “Announcement” indicts the exclusivity period should be held by the first ANDA filer, but the specific period is not clear.
5.Patent Term Extension / Compensation The protection term for invention patents is 20 years. For pharmaceutical patents, the length of clinical trials and drug applications lead to its patent exclusive period and payback period will be only a few years left, making it difficult to recover high R&D investment costs. For the aforementioned time loss, the “Opinion” provides that “the pilot appropriate compensation for the patent term of some selected new drugs may be given, in consideration of delay in the time for coming onto the market due to clinic trial, evaluation and approval”. It was announced in the "State Council Meeting” that “new drugs to be launched concurrently in China and globally will be entitled to a 5-year patent term extension”.
In conclusion, the Chinese government has demonstrated its determination to reform the drug patent linkage system with a view to improving the availability of drugs to the public. The perfection of the drug patent linkage system would result in a lasting benefit for drug companies, both branded and generic.
1 Liu Lichun, Zhu Xuezhong, <Choice of the Elements of Drug Patent Linkage System in U.S. and Canada and Its Inspiration to China>, Published in < Forum on Science and Technology in China>, Page 147, January 2014.
2 Article 18 of CFDA No. 28: “the applicant shall, in respect of the drug applied for registration or the prescription, technology, and usage applied, provide explanation on the patent and its ownership status of the applicant or others in China. If other people have a patent in China, the applicant shall provide a statement that there is no infringement to others' patent. The pharmaceutical regulatory departments shall publicize the explanation or statement submitted by the applicant on the website of the executive authorities. The relevant patent laws and regulations shall be applied to solve any patent dispute occurred during the drug registration process.”
3 Article 19 of CFDA No. 28: “For a drug with patent right obtained in China; another applicant may apply for registration within two years prior to the patent expiration. SFDA shall examine the application according to these Measures and shall issue the approval number, Imported Drugs Registration Certificate or Pharmaceutical Products Registration Certificate after expiration of the patent if the requirements are met.”
4 Article 20 of CFDA No. 28: “In accordance with the provisions of Article 35 of the Implementation Regulations, for a period of 6 years from the date of approval of the original applicant, SFDA shall not approve a
subsequent application that uses, without the consent of the original applicant, the undisclosed R&D data and other data generated by the original applicant approved to manufacture or market a drug containing new chemical ingredients unless the submitted data is generated by the subsequent applicant independently.”
5 At the 13th National People’s Congress of the People’s Republic of China, the Programme of Reforming the Institutions of the State Council (“Programme”) on 17 March 2018. According to the Programme, some
of the institutions under the State Council were abolished and reorganized, including the China Food and Drug Administration (“CFDA”) and the National Health and Family For the healthcare and pharmaceutical sector. A new drug authority, the China Drug Administration (CDA), was formed under the aegis of the State Market Regulatory Administration (SMRA) with the abolition of the CFDA.
6 Article 11 of the Patent Law: “After a patent right for an invention or a utility model is granted, except where otherwise provided for in the Law, no entity or individual may, without the permission of the patentee, exploit the patent, namely, for production or commercial purposes, to manufacture, utilize, offer for sale, sell or import the patented product thereof, or to use the patented process, or to utilize, offer for sale, sell or import the product directly obtained through the said patented process”.
7 See table 1 of the Classification.
8 Article 66 of “Administrative Measures for Drug Registration”: “In order to protect the public health, SFDA may set a monitoring period for the new drugs approved to be produced. The drug-monitoring period shall start
from the date of approval for production, and shall not exceed five years. During the monitoring period of a new drug, the SDFA shall not approve other enterprises' production, import or change of dosage form.”
9 Article XV of the Opinion: “The drugs newly approved for marketing or drugs passing the quality and efficacy consistency evaluation of generic drugs shall be included in the catalogue of drugs on the Chinese market, clearly specifying the attributes of innovative drugs, new improved drugs, and generic drugs of which the quality and curative effect are consistent with those of the new drugs, as well as the information such as the effective ingredients, dosage form, specification, marketing authorization holder, patent right and test data protection period.”
10 According to Article XIX of the “Opinion”, CDA should “list of drugs of which the patent right expires or is terminated or invalid, without application for generic drug, shall be released on a regular basis”.
11 See 21 U.S.C § 355(b)(1)(G) and 21 C.F.R. § 314.53(b).
12 FDA, Guidance for Industry 180-Day Exclusivity: Questions and Answers, available at https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM536725.pdf, last visited August 13, 2018.
13 Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (the “2003MMA”), §§1101,1102 (amending 21 USC §§355 (j)(2) and (5)).
14 See 21 U.S.C § 355(j)(2)(A)(vii): “a certification, in the opinion of the applicant and to the best of his knowledge, with respect to each patent which claims the listed drug referred to in clause (i) or which claims a use for such listed drug for which the applicant is seeking approval under this subsection and for which information is required to be filed under subsection (b) or (c)-(I) that such patent information has not been filed, (II) that such patent has expired, (III) of the date on which such patent will expire, or (IV) that such patent is invalid or will not be infringed by the manufacture, use, or sale of the new drug for which the application is submitted; and (viii) if with respect to the listed drug referred to in clause (i) information was filed under subsection (b) or (c) for a method of use patent which does not claim a use for which the applicant is seeking approval under this subsection, a statement that the method of use patent does not claim such a use. The Secretary may not require that an abbreviated application contain information in addition to that required by clauses (i) through (viii)”.
15 “35 U.S.C. § 271(e)(2) : It shall be an act of infringement to submit—
(A)an application under section 505(j) of the Federal Food, Drug, and Cosmetic Act or described in section 505(b)(2) of such Act for a drug claimed in a patent or the use of which is claimed in a patent….” …..
35 U.S.C. § 271(e)(4) : For an act of infringement described in paragraph (2)—
(A) the court shall order the effective date of any approval of the drug or veterinary biological product involved in the infringement to be a date which is not earlier than the date of the expiration of the patent which has been infringed,
(B) injunctive relief may be granted against an infringer to prevent the commercial manufacture, use, offer to sell, or sale within the United States or importation into the United States of an approved drug, veterinary
biological product, or biological product,
(C) damages or other monetary relief may be awarded against an infringer only if there has been commercial manufacture, use, offer to sell, or sale within the United States or importation into the United States of an
approved drug, veterinary biological product, or biological product…”
16 <Analysis Report on Patent Infringement Litigation Date under Jurisdiction of Beijing Courts 2017>, issued by IPHOUSE Judicial Data Research Center.
17 <Analysis Report on the status quo and tendency of the patent judicial protection in China from 2013 to 2017>, issued by IPHOUSE Judicial Data Research Center.
18 According to Article 18 of the “Interpretations of the Supreme People's Court Concerning Certain Issues on the Application of Law for the Trial of Cases on Disputes over Infringement on Patent Rights” (the “patent
Interpretations”): Where the right holder gives a warning to others regarding the infringement upon patent rights and the caveatee or interested party gives a written notice demanding the right holder to exercise the
right of action, if the right holder neither withdraws the warning nor files a lawsuit within one month after receiving such written notice or within two months after the written notice has been given, and if the caveatee or
interested party institutes a proceeding to the people's court requesting the people's court to confirm that its act does not infringe upon the patent right, the people's court shall accept the proceeding.
19 Guo Jianqiang, <Study on China’s Patent Invalidation System>, published in <Science Technology and Law>, page 948, issue 5, 2015.
20 Supra note 13.
21 <Analysis Report on Patent Infringement Litigation Date under Jurisdiction of Beijing Courts 2017>&<Analysis Report on Patent Infringement Litigation Date under Jurisdiction of Beijing Courts 2016>, issued by IPHOUSE
Judicial Data Research Center.
22 More specific periods are illustrated in the Article 5 and 6 of the “Draft Implementing Measures”.
23 Pursuant to “the “Announcement” (not effective), the experimental data on the drugs that succeed in challenging patents and have already come on the overseas market while first generic drugs come on the domestic market shall be protected for one and a half years.








